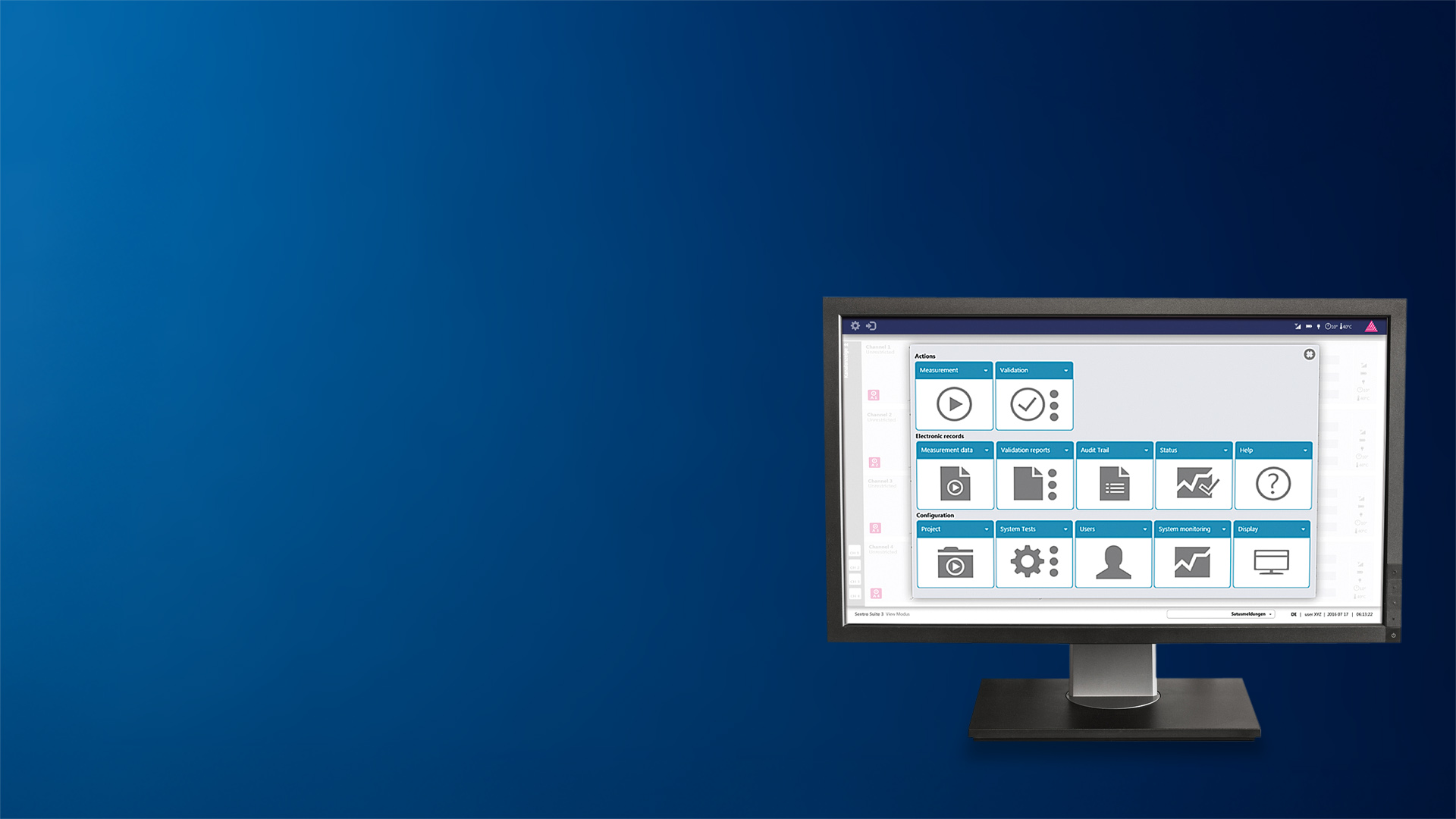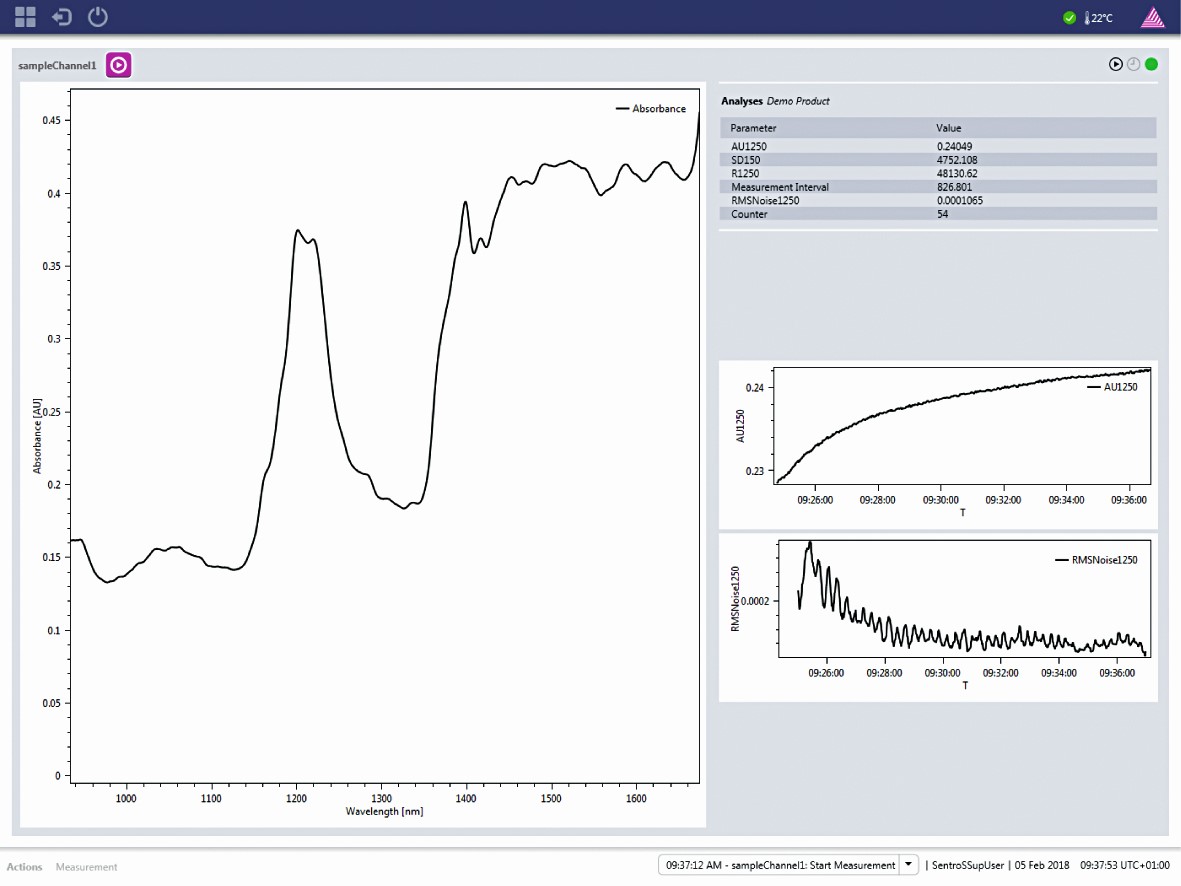
The SentroSuite GMP analyzer management software is the fully compliant operation platform for all SentroPAT analyzer types. SentroSuite enables all functionality of the systems in a flexible and configurable way. The user interface is intuitive and easy to use.
Main functions:
- Spectral data acquisition
- Configuration of system and measurement
- Wizard based system validation
- Generation of batch reports
- Analyzer health monitoring
Available interfaces:
- OPC-UA / OPC-DA server
- SIPAT
- SynTQ
- PharmaMV
- ProaXesS
User Management
The pre-configured user management supports all relevant roles. It allows flexible adaptation to the specific needs of the IT environment at the customer’s site. Windows domain integration is supported and allows assigning user groups to pre-defined roles in SentroSuite GMP as well as the full use of domain user accounts.
Audit trail
A secure and uneditable audit trail documents all system changes. All records do have a time stamp and the information on the user who made the change or performed an activity. Whenever applicable it also records the previous value together with the new value. The audit trail records can be easily exported to PDF.
File Security
All electronic records can be protected against any manipulation or deletion using a combination of user access control as a standard Windows functionality and proprietary software functionality.
Secured Mode
Data integrity is supported by a secured mode of operation. In this mode any access to the operating system level (e.g. TaskManager, Explorer etc.) can be denied for all users except those with administrative permissions.
Need personal assistance? We’re here to help!
Looking for more tailored support? Don’t hesitate to reach out—we’re happy to assist you! Or explore our knowledge base for in-depth insights and technical details.
Instrument validation
A full set of routines to verify the system performance according to USP<1856> and EP 2.2.40 is part of the software. These GMP relevant routines are completed
by additional test routines to verify the system is working within its specification at any time. A part of these additional routines allows performance verification,
while the system remains installed in the process.
Instrument health monitoring
The alarms and warnings generated by the system are configurable and include different error and warning conditions. They can beadapted to specific user requirements.
GAMP5 compliance
The complete software development lifecycle is governed by the quality system implemented at Sentronic, which fully implements all GAMP5 requirements.

Options for MVDA
- Statistical methods such as MBSD, Moving FTest, integral calculation and others
- Real-time predictions for PLS and PCA based on methods built in VektroDirektor or SIMCA
- Statisitcal methods for data such as mean, RSD, SD
- Correlation to target spectrum
- Coming soon: IOT
- Monitoring of peak positions, absorbance values and other simple monitoris
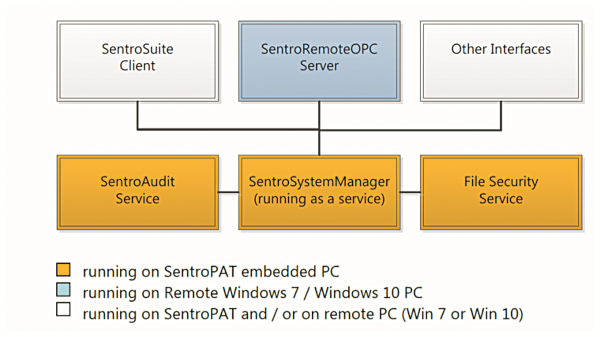
Basic setup using Windows Services
The entire functionality is based on the SentroSystemManager which is running as a service in Windows. It covers all basic analyzer functionality of the software. Running as a service it allows instrument operation with no user logged into the system. File security and audit service support the requirements for compliance with 21 CFR Part 11 and further GMP regulations.
SentroSuite, the graphical user interface but most commonly referenced as the analyzer management software, represents the client, which optionally can be installed on a remote computer to avoid competition for ressources on the SentroPAT embedded computer. The SentroRemoteOPCServer communicates via TCP/IP with the SentroSystemManager and can be installed remotely to avoid typical OPC configuration challenges.
Frequent Asked Questions
Learn more about SentroSuite GMP and how our analyzer management software can be used in a regulated environment.
SentroSuite is provided free-of-charge with each Sentronic instrument purchase. There is no routine user license fee.
Contact Sentronic. For users in development, we’d be happy to work with you to get a new version of SentroSuite installed on your system.
For customers working in highly-regulated, GMP facilities: we appreciate things are not so easy. We provided a list of what has changed with each version of SentroSuite and have a formal procedure for documenting updating a system’s software. We recommend pairing an update with an IOQ to provide test evidence that the system meets a site’s user requirements after installation of a new software version.
Short answer: Yes, we provide a certificate of 21 CFR Part 11 compliance with each SentroSuite installation.
Slightly extended answer: In our opinion a software can only support compliance to 21CFR part 11 by having dedicated funtions and capabilities. The final compliance needs proper installation and correct use. SentroSuite GMP offers a wide range of such functions but there might be some specific sections that are not covered. We're happy to discuss this in more detail.