When analytical chemists first encounter NIR spectroscopy in university, they are often ecstatic to find a method that doesn’t need any arduous sample preparation or just any caustic chemicals. NIR measurements in diffuse reflection mode are especially easy to implement: just shine a light at a sample and see what comes back. What could be possibly be easier?
Well, not so fast! While many pharmaceutical and food powders are easy to analyze using diffuse reflection probes, the diffuse reflection measurement process itself has some limitations. First, let’s take a look at what kind of light-matter interactions can occur with a loosely-packed powder:
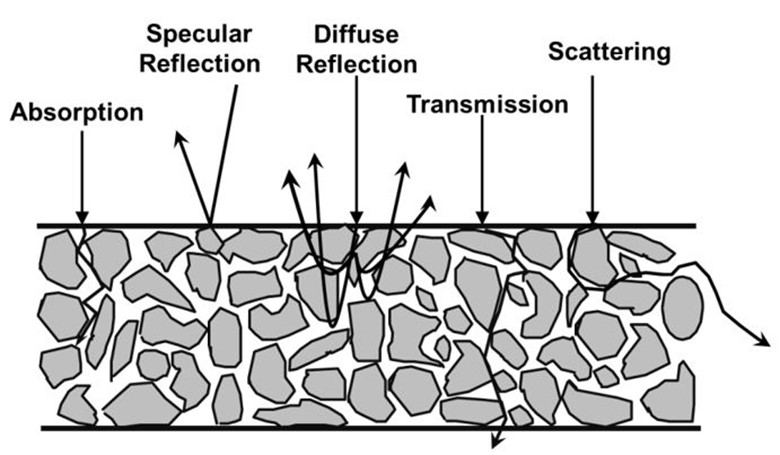
In diffuse-reflection measurement mode, we often assume that only absorption and diffuse reflection occur. This is not rigorously the case, but for many powders consisting of small, organic molecules, it’s a reasonable approximation.
We will often report NIR spectra in units of absorbance. We use a version of the Beer-Lambert Law to then derive relationships between NIR absorbance bands and concentrations of chemicals in the powders under assessment. The most used version of the Beer-Lambert law is:
A= ε b c
Where A is the absorbance at a given wavelength, ε is the molal absorptivity of a chemical, b is the pathlength of a measurement, and c is the concentration of the chemical. If we assume a fixed molal absorptivity and pathlength, this then means we have a linear relationship between our absorbance and concentration.
Unfortunately, we often can’t assume a fixed pathlength. This is because, in diffuse reflection mode, the pathlength of the measurement is dependent on both the wavelength of light and on the physical properties of the powder under assessment. Specifically, physical properties like bulk density and particle size affect how deep light can penetrate into a powder and, therefore, its NIR spectra measured in diffuse reflectance mode.

Is it then worth it to add a complicated assessment of bulk density and particle size distribution into our calculation of absorbance? No. Spectral preprocessing methods, as well as the use of empirical models like partial least squares (PLS), often means that we’re still able to derive chemical information from NIR spectra measured in diffuse reflectance mode. However, new NIR users should be aware of this phenomenon when analyzing samples. If you’re building a model to control a process that might have a lot of bulk density and particle size variability, that variability should be kept in mind when developing an NIR-based control.
