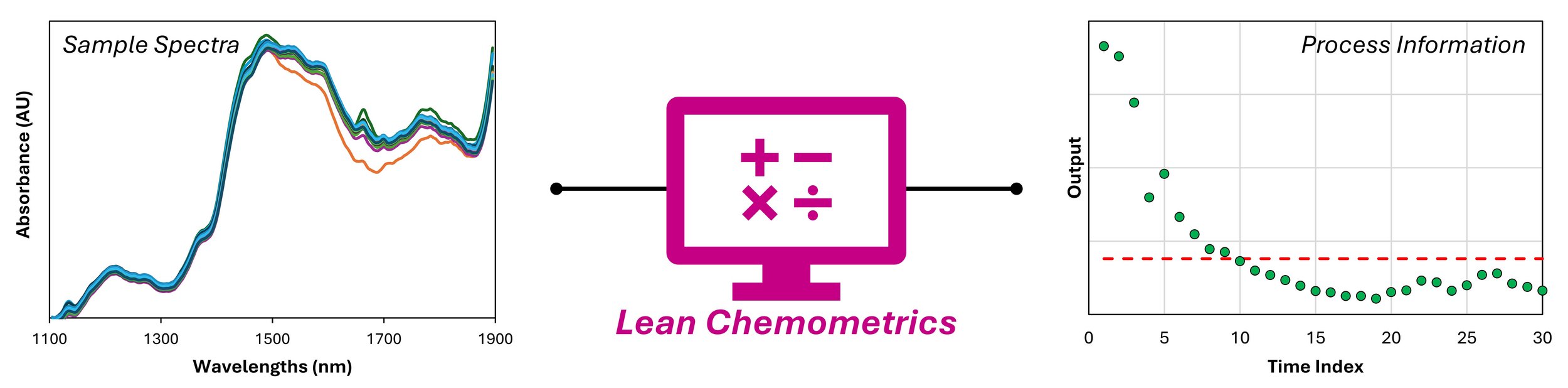
Introduction
There are many chemometric techniques to choose from when selecting how to convert your NIR spectra into process information. The choices range from the classic options like partial least squares (PLS) regression to cutting-edge machine learning methods. A major factor in the pharmaceutical industry influencing which chemometric technique to select is the required calibration burden, as we discussed last time. For many applications, the required number of calibration samples needed to implement conventional chemometric techniques can inhibit the desire to deploy PAT. In these cases, a class of techniques that we refer to as lean chemometrics become an appealing and viable alternative.
Qualities of Lean Chemometric Techniques
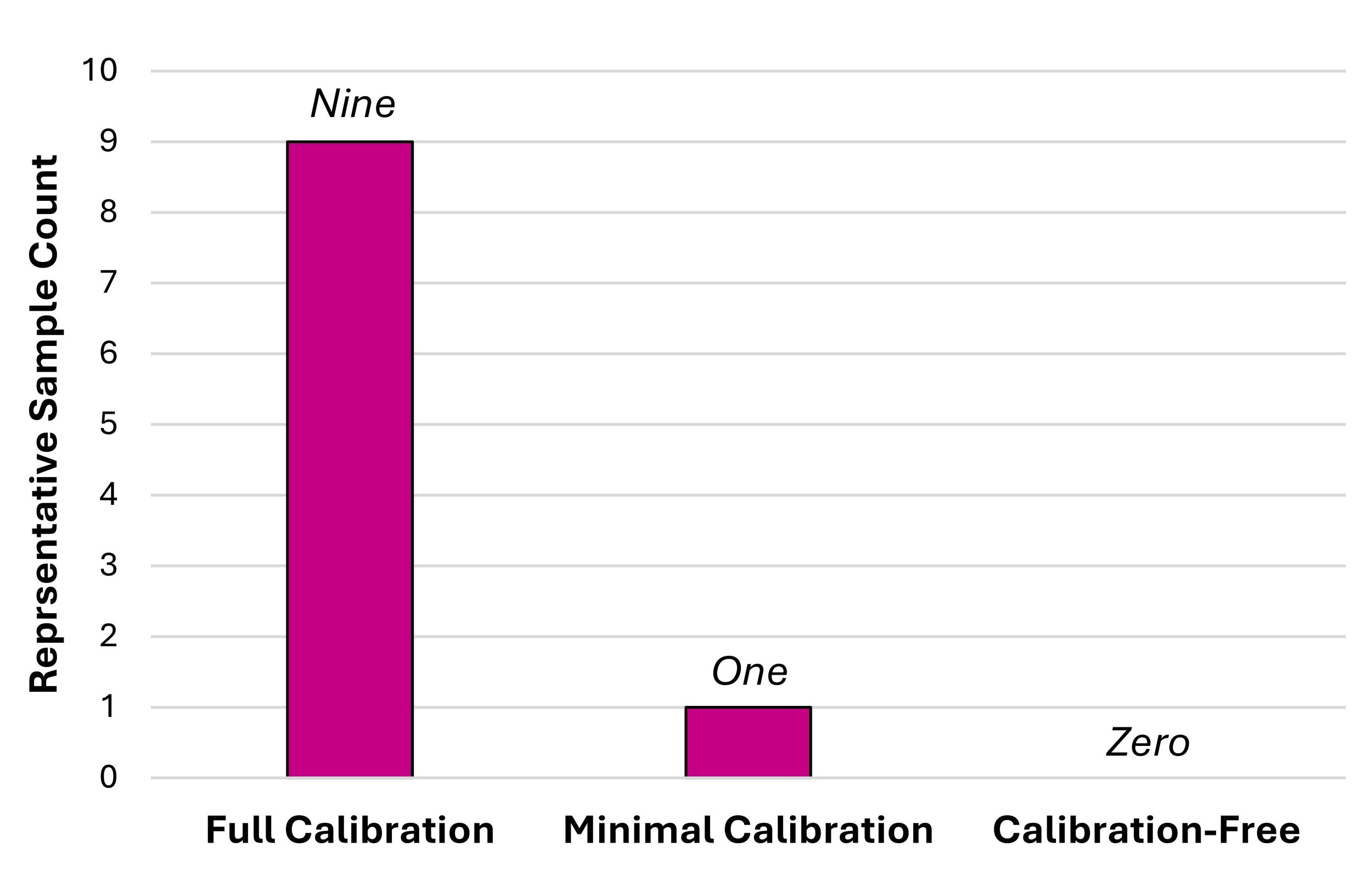
Lean chemometric techniques are multivariate data analysis (MVDA) methods conducted in a way that minimizes the drivers of calibration burden: time, material, and financial costs. In practice, this translates to using MVDA methods that require the least number of representative samples with corresponding reference data for calibration as possible. If at least one representative sample is required for calibration, the technique is considered as minimal calibration. On the other hand, if an interpretable output can be generated without representative samples, the technique may be referred to as calibration-free.
The moving block standard deviation (MBSD) analysis is a good example of a qualitative calibration-free method, as it only requires the parameterization of the block size to provide a trend of spectral variance in time-series data. Likewise, pure component methods are suitable as quantitative lean chemometric techniques, with only the pure component spectra of the individual chemical constituents required to provide analysis of mixture spectra.
Lean Chemometrics & Pharmaceutical PAT
Lean chemometric techniques are primarily applicable as fit-for-purpose methods for analyzing spectroscopic data of low complexity samples taken from highly characterized and well-controlled processes. Fortunately, this is an excellent description of many pharmaceutical applications of NIR spectroscopy as PAT. The lower calibration burden of developing lean chemometrics techniques enables their deployment even in circumstances when PAT would not necessarily be considered. For example, during early drug development stages, the limited availability and often extremely high cost of synthesizing the new API inhibits the development of a full calibration dataset for a PLS model with NIR PAT. However, the pure component spectra of the API and excipients are trivial to obtain, allowing pure component methods, like iterative optimization technology (IOT) algorithms, to serve as the MVDA method for NIR spectra interpretation and the PAT to be deployed in early manufacturing investigations.
There are a variety of lean chemometric techniques commonly utilized for spectral analysis in pharmaceutical NIR PAT applications. The MBSD analysis and moving block F-test (both available in SentroSuite) are popular ways to assess blending end point and affirm blend uniformity. The interest in pure component methods has also increased recently, due to multiple scientific publications demonstrating similar quantitative performance between these lean chemometric techniques and conventional PLS approaches.
The pharmaceutical industry is eager to reduce the financial cost of drug development and quicken their timelines to get new drugs to market. Spectroscopic PAT is an essential part of that vision as it is an established and powerful strategy to obtain deep processing understanding and achieve process control. Lean chemometrics can effectively enable the spread and application of spectroscopic PAT by reducing calibration burden significantly.
